The CIP (Cleaning-in-place) process is responsible for cleaning the industry’s equipment, without having to disassemble it. In the food industry, these systems work through the use of inline process measures, which are used to control the cleaning temperature, flow, and concentration of detergent in real time. This contributes to optimization, being responsible for saving natural resources such as water and energy.
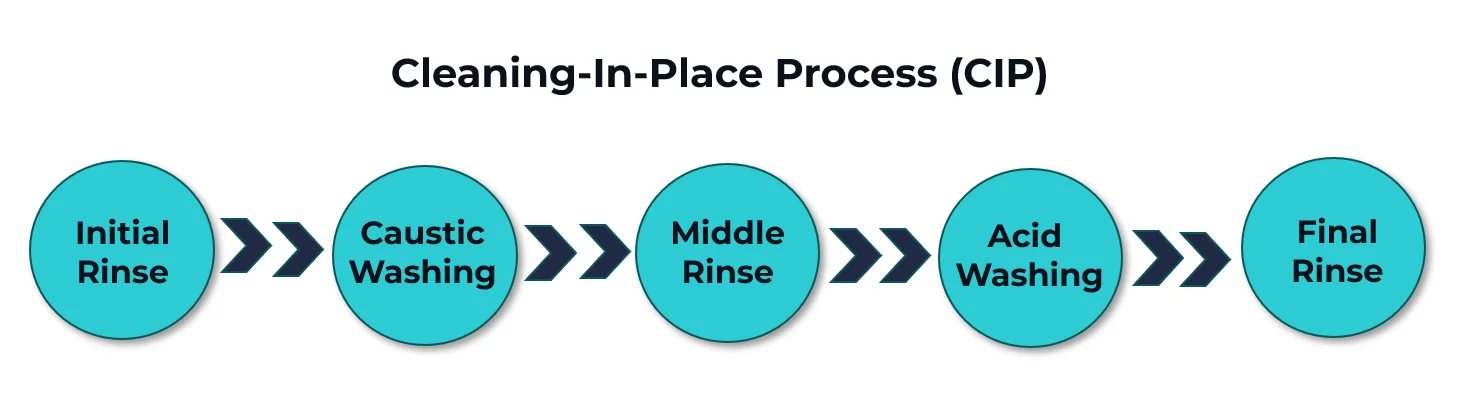
It is mandatory in sectors such as oil and food, but especially in those that are highly regulated in terms of quality and safety. CIP is done through cycles, and its time varies, according to the product produced in the different lines. The order of the steps may vary, but in general, the CIP process encompasses:
- A pre-rinse made with water treated by reverse osmosis, performed in three repetitions, of 1 minute each, to remove the coarser dirt;
- Caustic washing of an average of 30 minutes to dissolve organic and inorganic waste. The solution uses products such as caustic soda, phosphoric and nitric acids, and sodium hypochlorite (hypo);
- 1-minute intermediate rinse , also with treated water;
- Disinfectant washing, lasting up to 10 minutes. This solution uses products such as sodium hypochlorite, 70% alcohol, and peracetic acid (PAA), and kills resistant bacteria and microorganisms, such as Escherichia coli;
Different types of CIP
In addition to the general process described, the CIP has several variations, according to its configuration, level of automation and quality. Some examples are:
- Single-shot CIP: In this type of cleaning, the chemical solution is used only once and goes directly to the drainage;
- Recovery CIP: Cleaning products are stored after use, treated and reused in subsequent cycles;
- Multi-channel: This process has multiple channels for cleaning different parts of the equipment simultaneously;
- Fixed or mobile systems: fixed systems are integrated into the production line, while mobile systems can be adapted as needed;
- Centralized and distributed processes: A centralized CIP system is a single system that provides cleaning solutions for the entire process facility. It can provide many different circuits and coordinate a large number of operations from a single location. A distributed system means that individual sections of the plant can be cleaned with a dedicated local unit.
In the pharmaceutical industry, the most widely used cleaning method is the Single-Pass CIP. The choice is justified, as this type is aimed at cleaning heavy dirt. Specifically, this industry uses the single-shot process to clean Active Pharmaceutical Ingredients (APIs), excipients, mist, and tablet coatings from equipment. The cleaning solution circulates only once through the system and should be discarded. This is particularly useful when cleaning residues must not come into contact with any other type of material.
In the chemical industry, CIP systems must be designed to withstand the harsh chemicals and high temperatures associated with processing them. Cleaning solutions should be carefully selected to ensure that they do not react with any other chemical ingredients.
How to optimize the CIP process
During CIP, some aspects can hinder the efficiency of the process, which may vary depending on the type of industry. In the beverage industry, for example, it can be longer and require the use of more chemicals. This is because drinks such as soda have sugar in their recipe, which makes cleaning difficult due to their high adhesion to surfaces.
The CIP cycle is highly used precisely because it is automated, but some tasks still require human intervention. Regular system maintenance, calibration of sensors, and replenishment of chemicals may require manual actions. Data science is a great ally in these cases, by providing information that allows these tasks to be performed in a planned and optimized way.
On a production line that uses liquid inputs, equipment often includes complex valve pipes and dies. Mapping and analyzing matrix data optimizes the CIP cycle. Data science applied to this equipment also contributes to other factors, such as:
- Time: Having control of CIP time has a positive impact by identifying which processes are within or outside the parameters, and in which ones need improvement;
- Water and energy: These resources are essential in industry, and their monitoring allows for the reduction of their consumption;
- Cleaning solutions: Monitoring the amount of chemicals used results in cost savings for companies, as these can be costly;
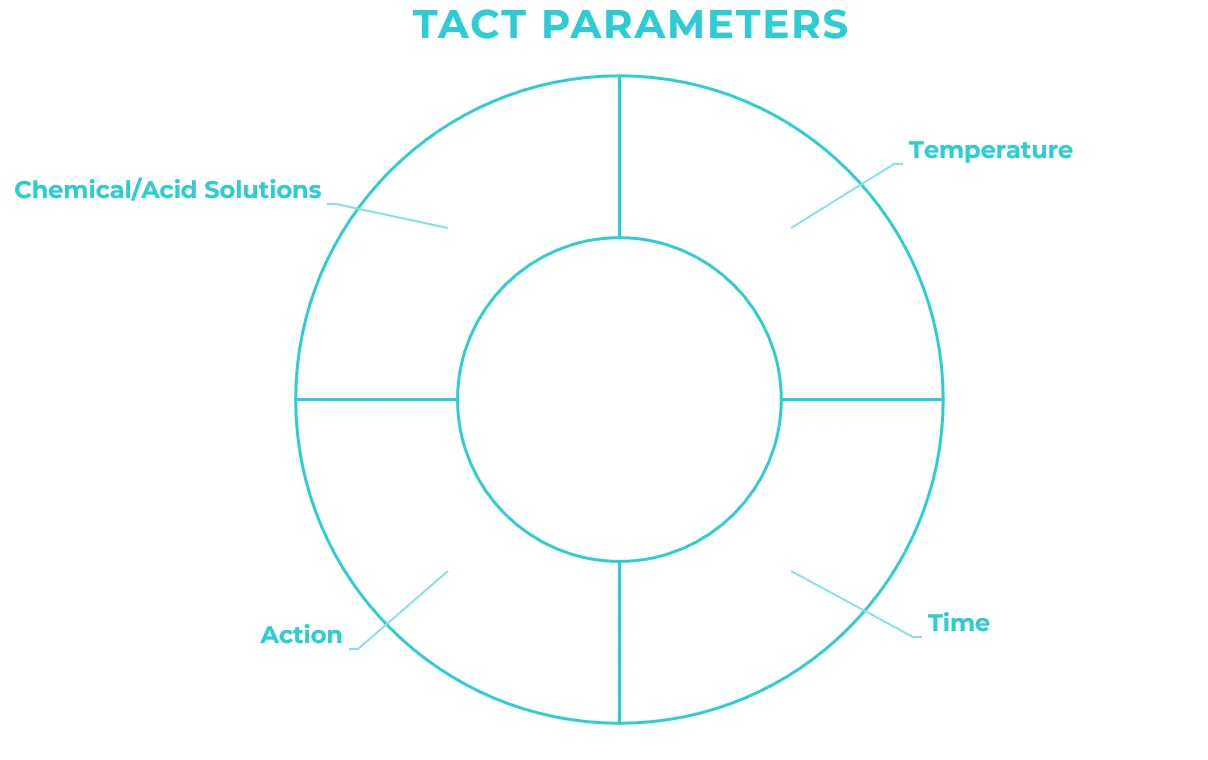
It is important to emphasize that the elements used in the CIP have equal importance and participation in the process. The temperature of the product and the contact time with the surface help to eliminate residues more quickly. Just as the right combination of the chemical/acid solution results in a more effective action.
Supervision of the CIP process
The parameters for inspecting the CIP process in the industry are very strict and involve several steps. This is because a poorly executed CIP has a significant impact on several areas, including the quality of the final product. In the beverage industry, if cleanliness standards are not executed correctly, there is a risk of microbiological and chemical contamination of products. This compromises security, affects customer satisfaction, and can even lead to recalls.
The Brazilian Government inspects the CIP process through the Ministry of Agriculture, Livestock and Supply (MAPA). The evaluation of sanitary conditions is done through videoscope analysis of the system and the sizing of equipment according to national and international standards. Internally, Brazilian industries audit cleaning through the correct sizing of TACT parameters, based on the typology of the processes.
The United States also oversees these safety and quality parameters. Manufacturing industries must comply with the strict rules of the FDA (Food and Drug Administration), responsible for promoting public health through food supervision. Within the CIP, an example of this is the definitive registration of which cleaning products and disinfectants should be used in the food industry.
Worldwide, other guidelines include the EHEDG (European Hygienic Engineering & Design Group), the 3-A Sanitary Standards and the ISBT (International Society of Beverage Technologists). They provide detailed guidance on the management, validation and design of CIP installations to ensure that validated elements meet sanitary requirements.

Data analytics as part of the future of CIP
According to a survey published by Transparency Market Research (2019), the CIP process is projected to have an annual growth of 15.7%. This suggests that with this investment by the industry, factories that do not make use of CIP may be at a competitive disadvantage.
The justification for this growth is the industry’s interest in sustainable strategies, which prioritize efficiency and a better relationship with the environment. Industries that aim for eco-friendly practices, as a result, are also investing in the implementation of CIP processes that reduce water and energy use. Also, the increased concern about health issues, such as food poisoning and cross-contamination, has encouraged the food industry to invest more in CIP.
The integration of the CIP process with technologies such as data science is also revolutionizing the industry. To do this, it is possible to cross-reference variables such as time and speed, to identify conductivity problems or equipment maintenance. Data collection and analysis help ensure smooth flow of operations, reduced manual work, and more consistency during the CIP cycle.
Another trend is the use of innovative transition sensors, which determine when one phase of the cycle ends and when the next one should begin. In addition, the industry is increasingly seeking to meet new market demands, and for this it is seeking to develop new products and recipes. To keep up with this pace, processes are also updated, including cleaning, which is increasingly modern and efficient. Learn more about ST-One.
Check out the CIP Process in a digitalized industry:
